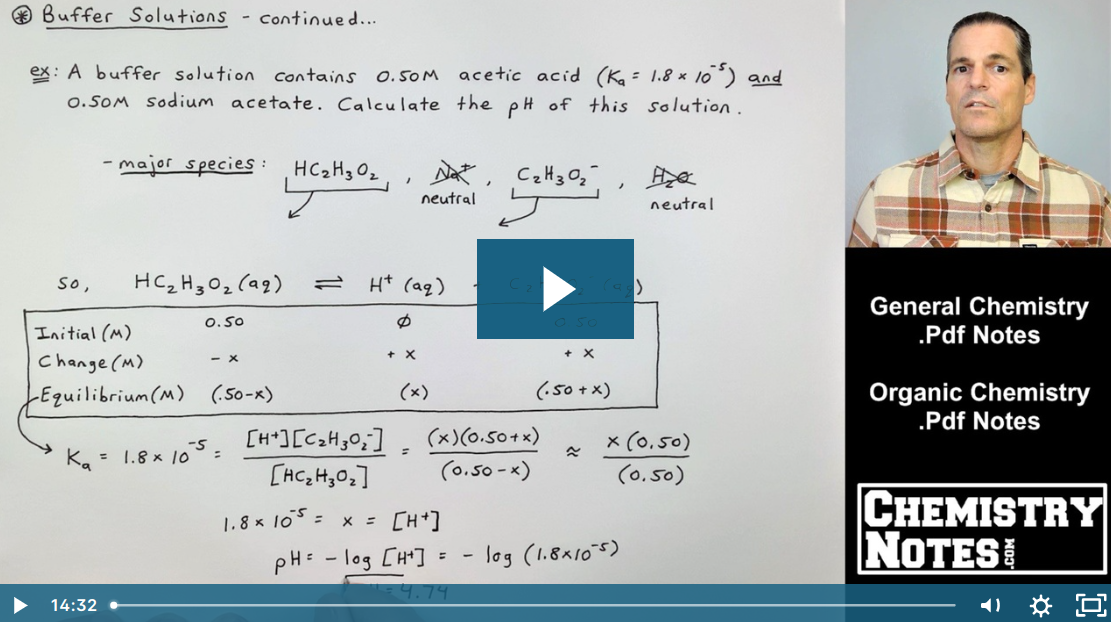
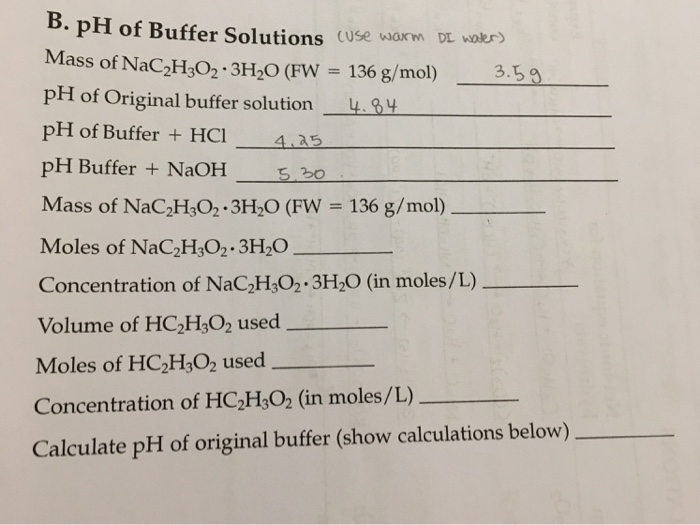
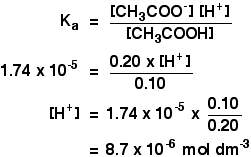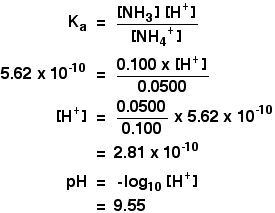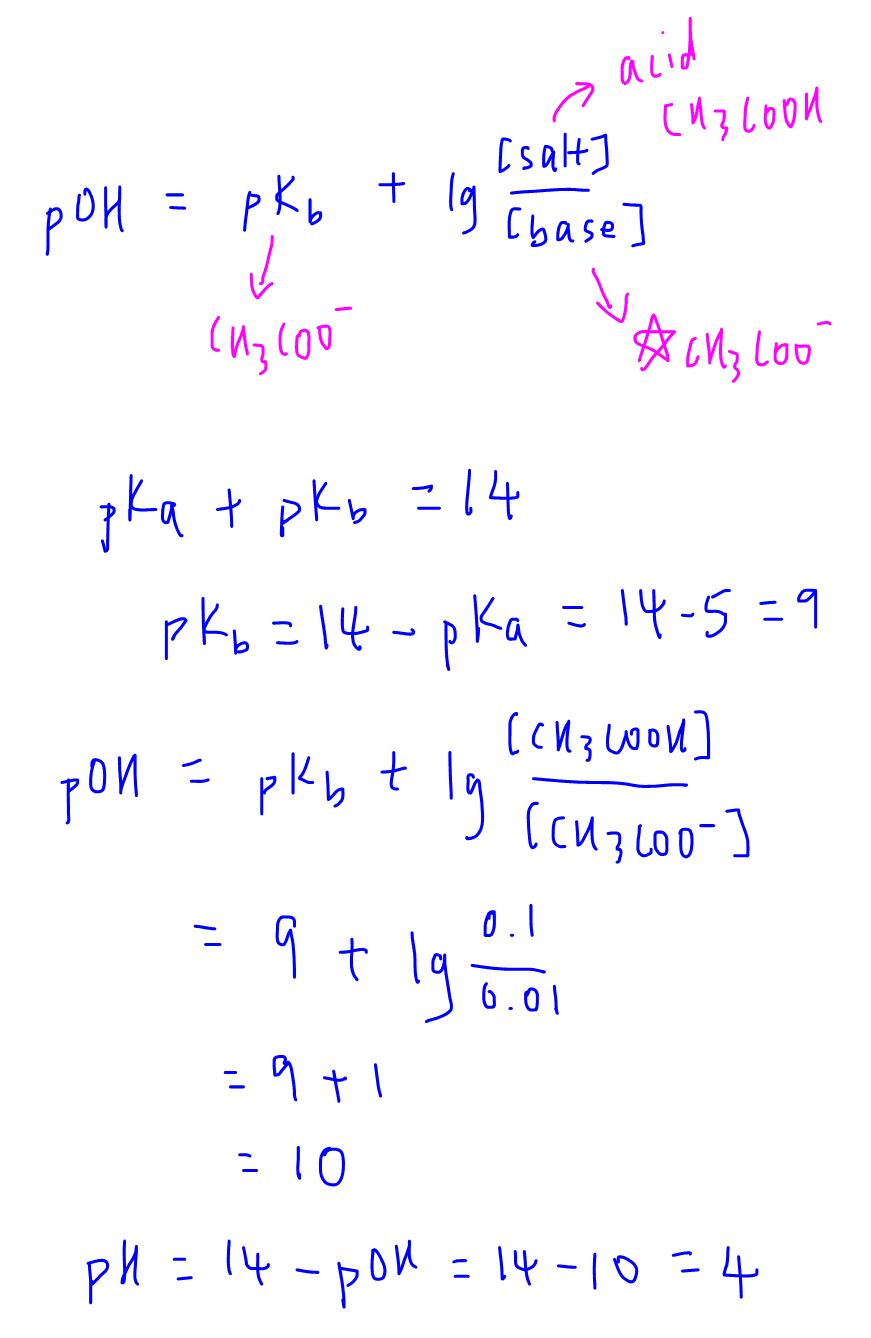SOLVED: B. pH of Buffer Solutions '9. 50 9 Mass of NaCzH;Oz * 3HzO (FW 1365 g/mol) 41 pH of Original buffer solution of Buffer + HCI 4S pH pH Buffer +

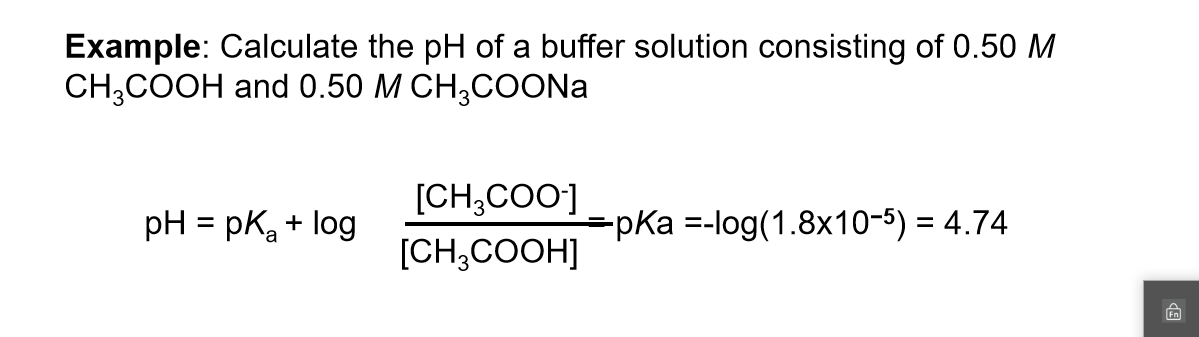
Acid-Base Buffers Equation & Examples | How to Calculate pH of a Buffer - Video & Lesson Transcript | Study.com
![Calculate the pH of a buffer solution prepared by dissolving 30g of Na2CO3 in 500 mL of an aqueous solution containing 150 mL of 1M HCl . Ka for: HCO3 = 5.63 × 10^-11 [ log 133150 = - 0.05 ] Calculate the pH of a buffer solution prepared by dissolving 30g of Na2CO3 in 500 mL of an aqueous solution containing 150 mL of 1M HCl . Ka for: HCO3 = 5.63 × 10^-11 [ log 133150 = - 0.05 ]](https://dwes9vv9u0550.cloudfront.net/images/6404140/adc2d8b1-954e-4b3c-b9c4-733c3311ce8f.jpg)
Calculate the pH of a buffer solution prepared by dissolving 30g of Na2CO3 in 500 mL of an aqueous solution containing 150 mL of 1M HCl . Ka for: HCO3 = 5.63 × 10^-11 [ log 133150 = - 0.05 ]

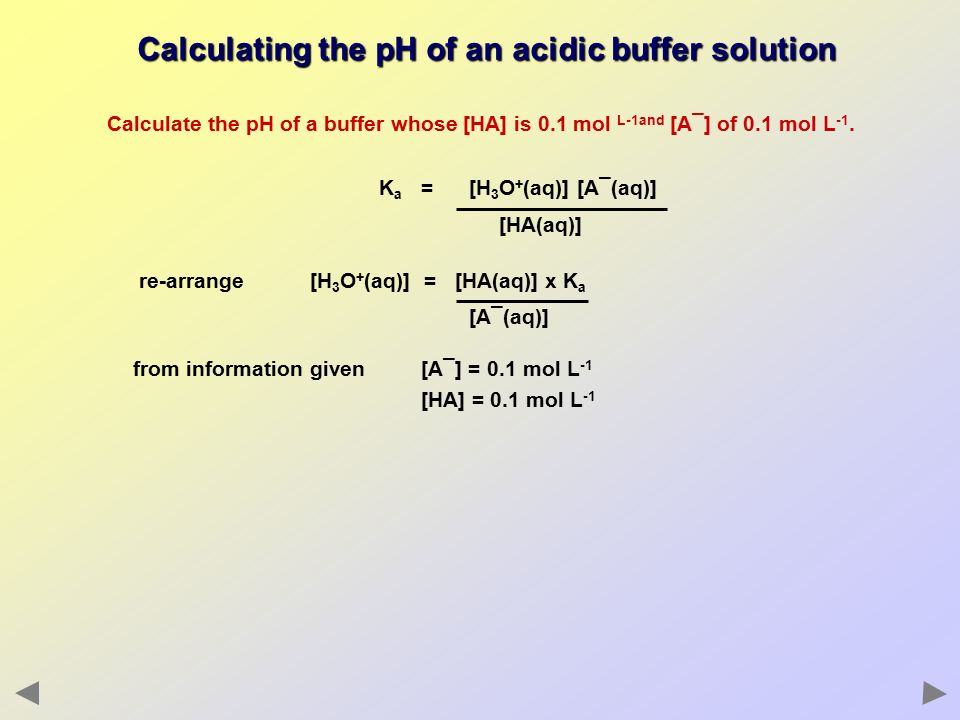
Calculate the pH of buffersolution containing 0.05 mol NaF per litreand 0.015 mol HF per litre. [K= - Brainly.in