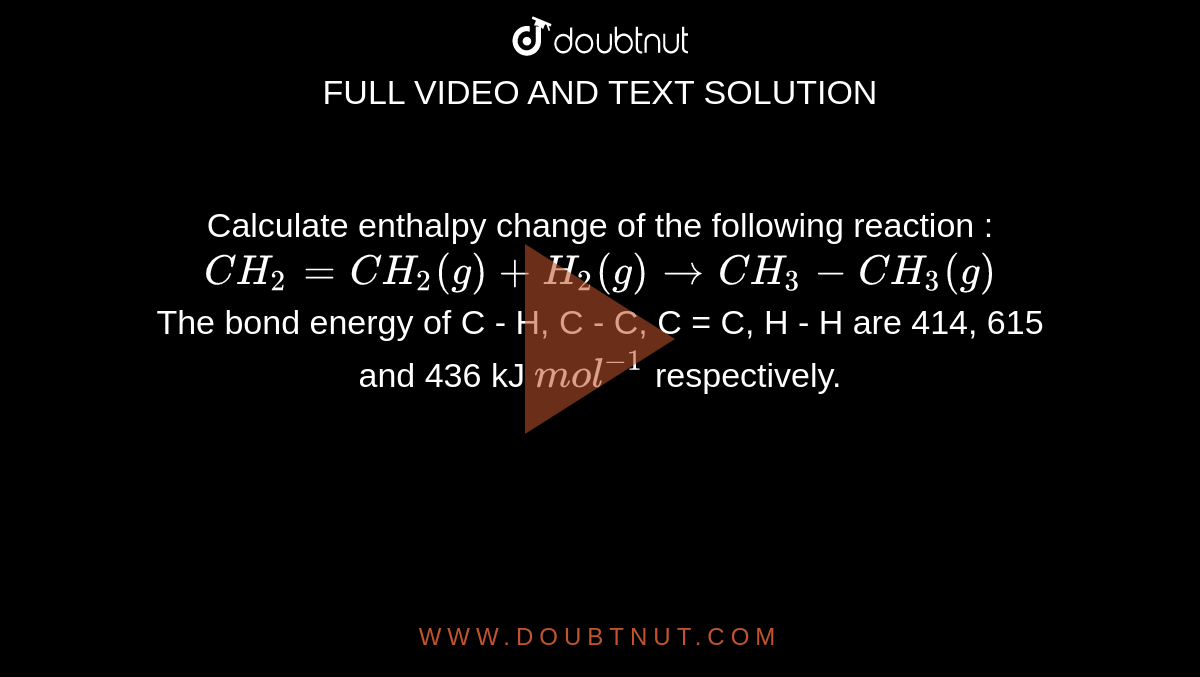
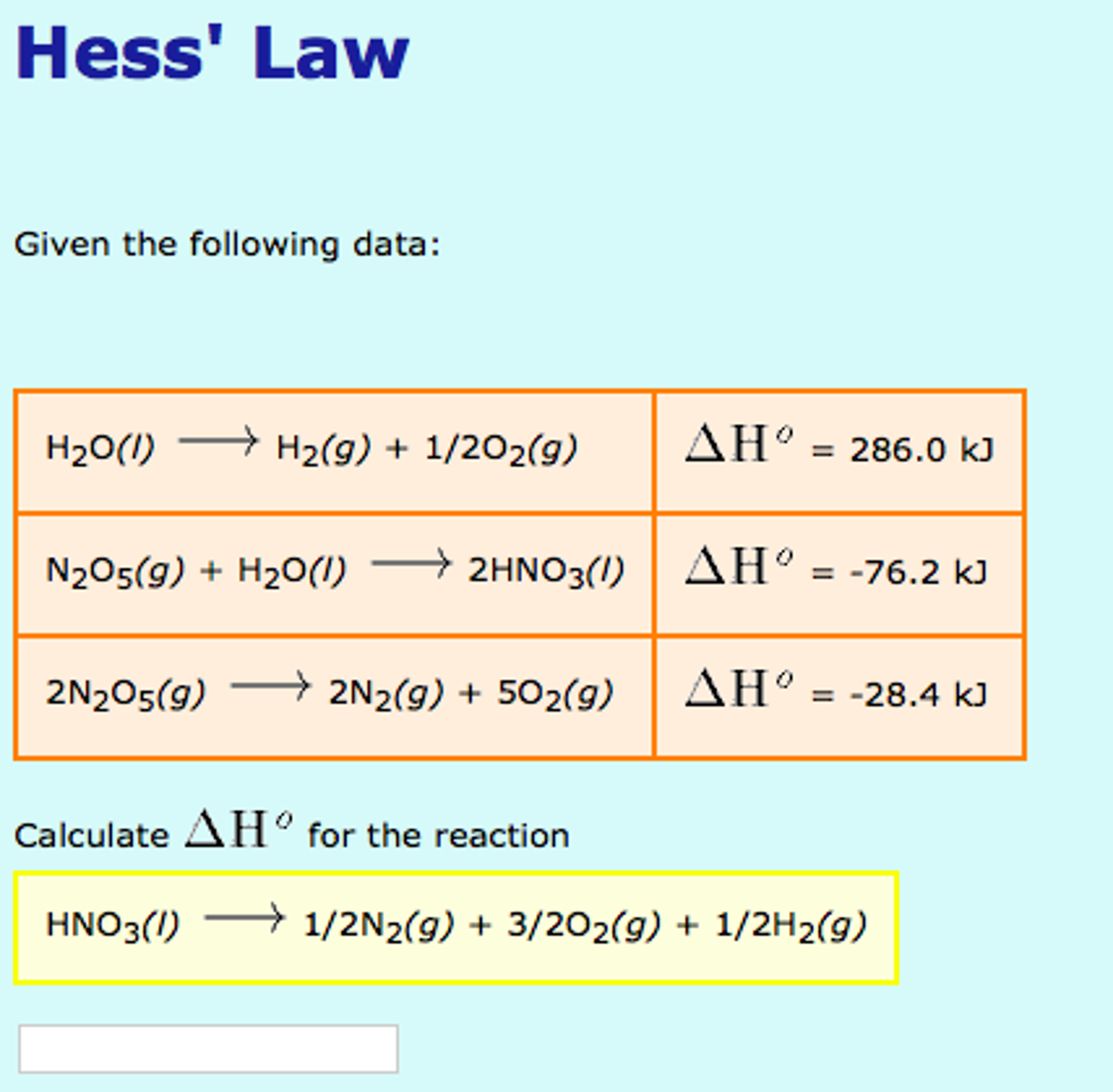
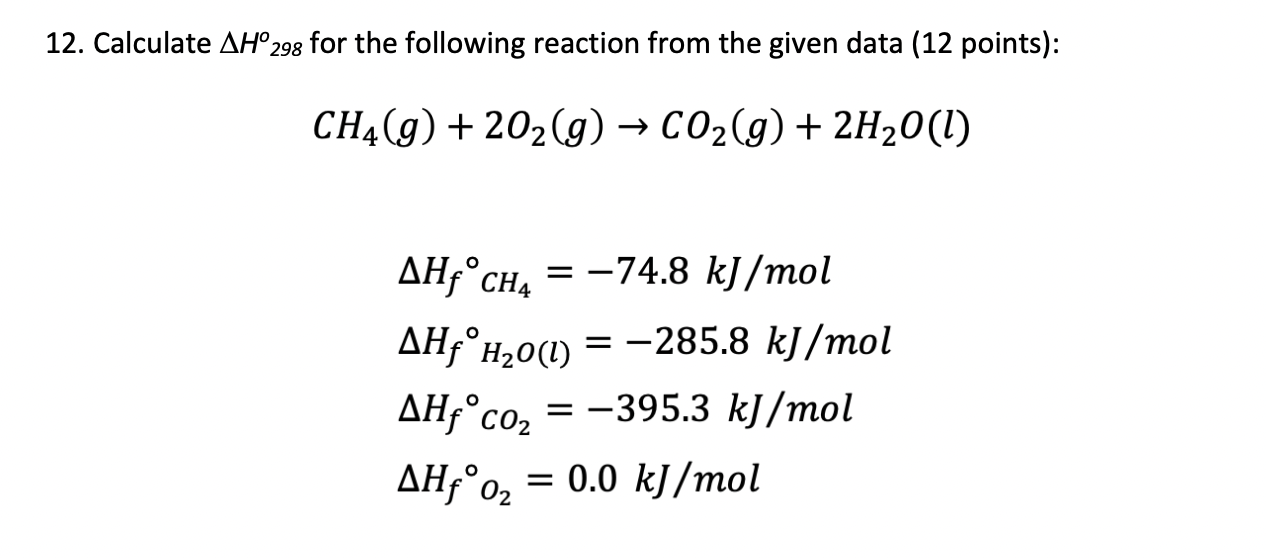
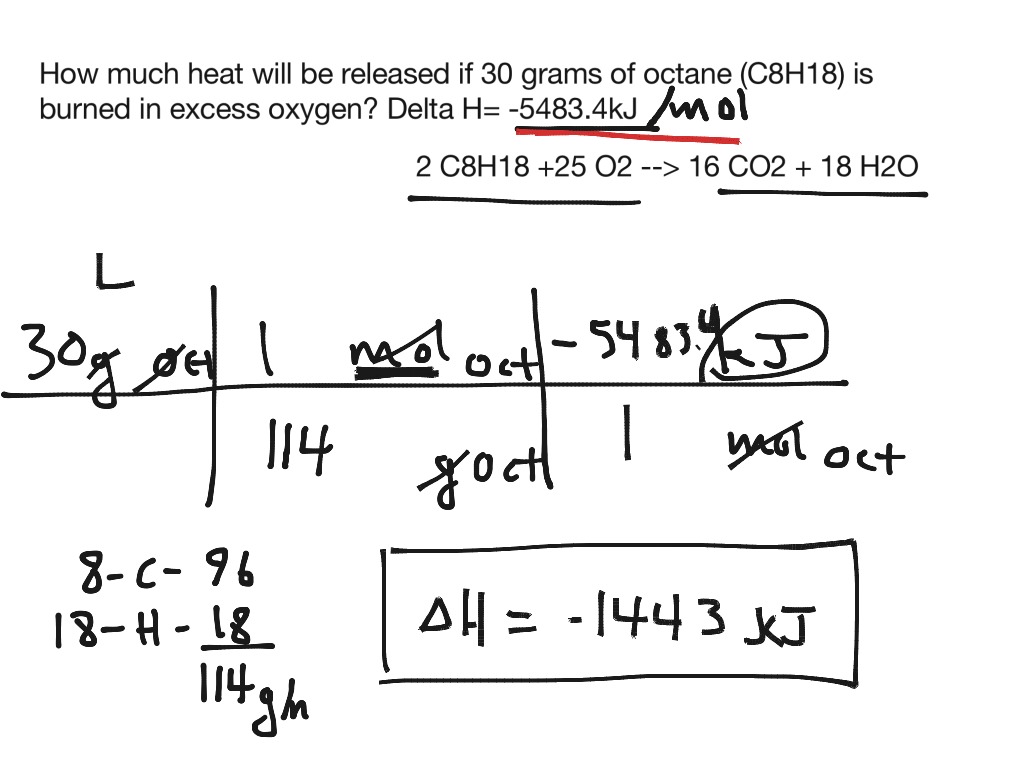
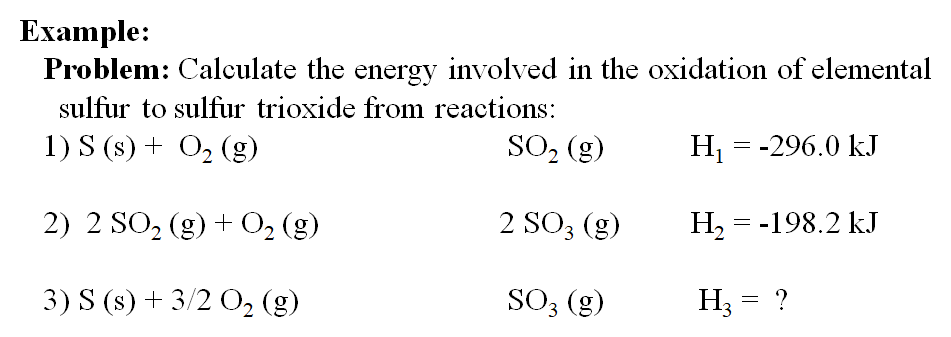Given : NO(g) + O2(g)→ NO2(g) + O2(g) H = - 198.9 KJ/mol O2(g)→ 2O2(g) H = - 142.3 KJ/mol O2(g)→ 2O(g) H = + 495.0 KJ/mol The entalpy change ( Δ
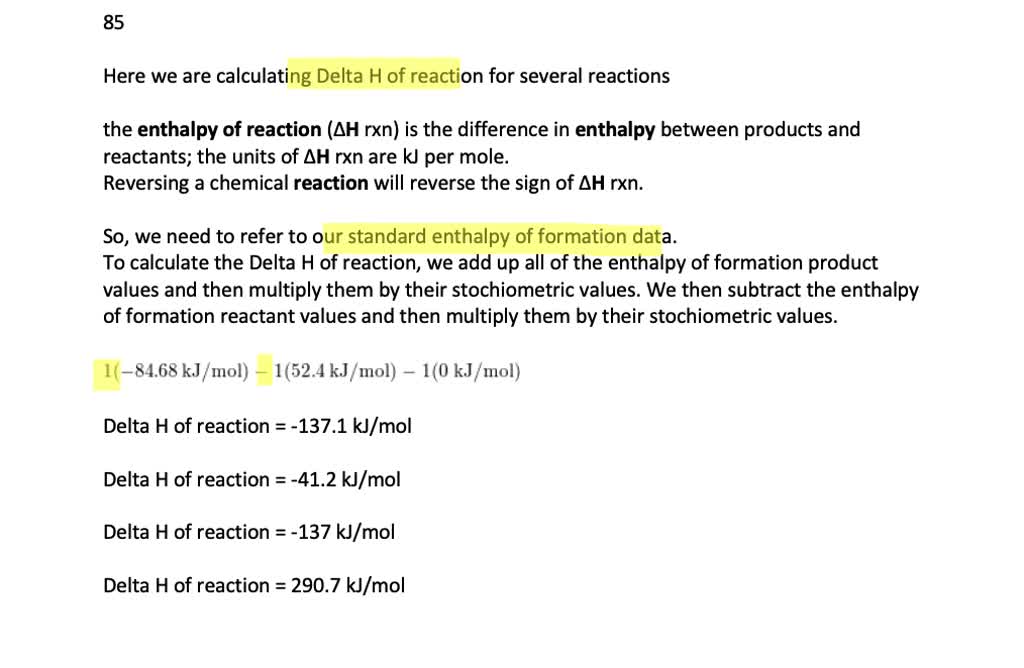
SOLVED:Use standard enthalpies of formation to calculate ΔHrxn^∘ for each reaction. a. C2H4(g)+H2(g) ⟶C2H6(g) b. CO(g)+H2O(g) ⟶H2(g)+CO2(g) c. 3 NO2(g)+H2O(l) ⟶ 2 HNO3(a q)+NO(g) d. Cr2O3(s)+3 CO(g) ⟶ 2 Cr(s)+3 CO2(g)
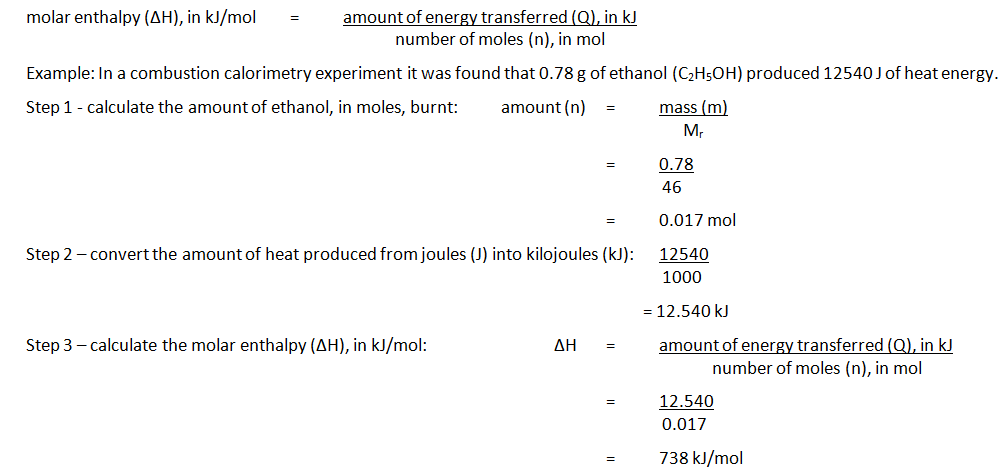
3:04 calculate the molar enthalpy change (ΔH) from the heat energy change, Q - TutorMyself Chemistry

Question Video: Calculating Standard Enthalpy of Combustion of Methane Using Standard Enthalpies of Formation of Methane and Carbon Dioxide | Nagwa

OneClass: Use the Delta H degree f information provided to calculate Delta H degree rxn for the foll...

⚗️Hess's law A -> B Delta H= +30kJ B -> C Delta H= +60kJ A.) Use Hess's law to calculate the - Brainly.com